Medical Hypoallergenic Wound Adhesive Dressing Protector Wound Care Dressing Products
Medical Hypoallergenic Wound Adhesive Dressing Protector Wound Care Dressing Products
Instructions for foam pressure ulcer dressing(self-adhesive)
1. First clean the wound around the skin, try to remove the dead skin surrounding the wound, and the helper should clean their hands to prevent extraneous bacterial infection.
2. When using it, please take the foam pad side towards the wound, gradually tear the release paper, adjust the wound site to stick, and then continue to peel half of the release paper until the dressing is pasted completely and smoothly on the wound.
3. It is a normal phenomenon that the dressing becomes swelled and white after absorption and swell. If any leakage, please immediately replace the dressing.
Foam pressure ulcer dressing(non-self-adhesive)
1.Non-self-adhesive foam dressing is required to fit with sterile gauze and bandage when used;
2. Non-self-adhesive foam dressing is not waterproof, and the sore condition is unable to be observed in time due to isolation from gauze.
3.This product can be used and fixed under a nurse's guidance.
Ultra-thin hydrocolloid dressing
1. Application scope: for the surface of the sore and wound with no obvious exudate
2. Function: absorb little exudate, keep the sore and wound moist, promote granulation growth
3. Instructions: refer to hydrocolloid foam dressing
| Product name | hydrocolloid foam pressure ulcer dressing from Celecare |
| Brand | Celecare |
| Model | B0810 |
| Specification | 15×15cm, dressing 10×10cm |
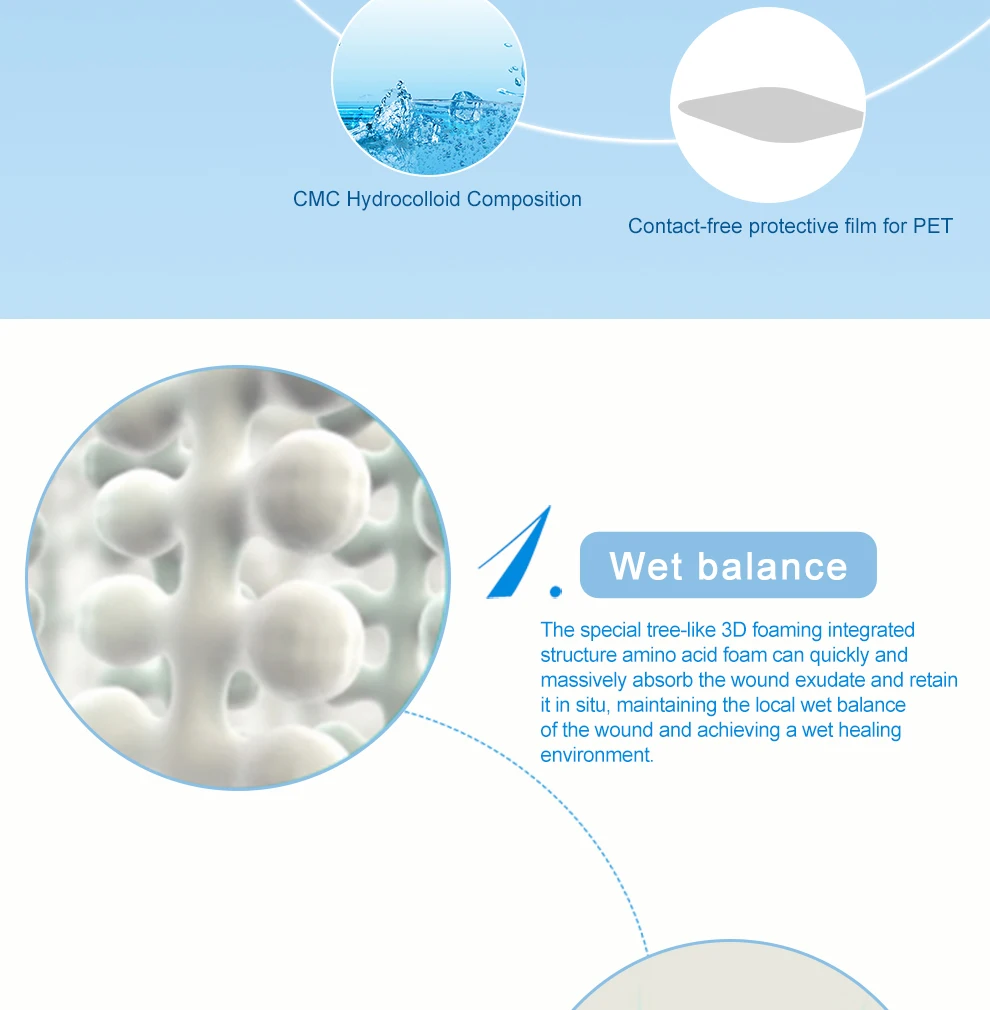
| Material | 3-D foamed polymer material, CMC hydrocolloid |
| Sterilization method | ionizing radiation sterilization |
| Expiry date | three years |
| Application scope | all kinds of high permeable wounds, bed sores, wound of donor site and scratch, etc. |
| Product advantages |
A.Semipermeable waterproof film, get rid of air, effectively prevent wound infection B.Imported dressing, comfortable and breathable, soft and elastic, combined with a pressure bandage, comfortable without causing wound irritation C.CMC hydrocolloid component, inward swelling, more fitting to the wound surface D.Absorb rapidly large amounts of exudate, preserve exudate in original position, keep the wound moist |
| How often should the dressing be changed? | Changing depends on the amount of wound exudate and the condition in which the dressing used is. Usually the dressing is replaced once every 1 to 3 days, or if the exudate exudes close to a 2cm edge of the dressing please replace the dressing in time. This product can be replaced for up to 7 days. |
| Note | A.If a patient has a systemic or local infection, but the infection is under control, please use this product under a doctor's guidance. If the wound is produced by arterial insufficiency, please follow a doctor's or a nurse's guidance, and the wound should be examined every day. B.Before radiotherapy(including X-ray, ultrasound, hyperthermia and microwave therapy), please remove this product. This product can not be used with saline and hydrogen peroxide. |











Celecare Medical Wenzhou Co, Ltd was founded in 2009 with registered capitalof 5 million, an enterprise specializing in researching, developing and manufacturingmedical products, which are popular with domestic and foreign customers. We haveexported our products to the United States, India, the United Kingdom, France, Sou-th Korea, Turkey, Russia, Africa, South America and the Middle East. Our factory isconveniently located within half an hour s drive from Wenzhou airport and high-speed railway station, covering 2, 400 square meters(1000 square meters for a 100, 000class purification workshop), with more than 50 employees. We have got certificatio-ns related with the quality management system, including CE, FDA and ISO13485We serve warmly our customers and operate with good faith. Welcome friends fromall walks of life to visit Celecare Medical Wenzhou Co, Ltd for guidance, businessdiscussion, cooperation and mutual benefits.


1.Can I get some samples?
A.We are honored to send you our samples, but the express fee need to paid by youresteemed company.
2.Can you do custom-made?
A.OEM. ODM welcomed.
3.How about the delivery time?
A.15-25 days, it is based on your QTY.
Contact
Supplier